Leave Your Message
Request a Quote
Laboratory Ultrafiltration has become a crucial method for efficient separation processes in various industries. According to a recent report by Smith & Williams Research, the ultrafiltration market is expected to grow by 10% annually. This technology is widely favored for its ability to filter particles in the range of 1 to 100 nanometers. This precision allows for applications in biopharmaceuticals, water treatment, and food processing.
Dr. Emily Tran, a leading expert in laboratory ultrafiltration, states, "The precision in particle separation is unmatched, paving the way for innovative solutions." However, despite its advantages, many laboratories struggle with optimizing their ultrafiltration systems. The choice of membrane material, configurations, and operating pressures can significantly impact results. It's essential to note that not all setups yield the same effectiveness, which raises questions about best practices.
Laboratory ultrafiltration offers substantial benefits, yet challenges persist. Many professionals in the field may overlook optimization techniques, potentially hindering their results. Continuous research and feedback from experts like Dr. Tran are vital to advance this technology further. Addressing these issues is crucial for achieving the highest efficiency and reliability in laboratory ultrafiltration applications.

Ultrafiltration is a crucial technique in laboratories for separating molecules based on size. This method employs a semi-permeable membrane that allows smaller molecules to pass while retaining larger ones. It’s widely used in various applications, including biotechnology, food processing, and water treatment. Understanding the principles behind ultrafiltration can enhance its effectiveness and broaden its applications.
When using ultrafiltration, consider the membrane properties closely. Different materials possess varying pore sizes and chemical compatibilities. This choice can significantly influence separation efficiency. Adjusting factors like pressure and temperature can also optimize the process. For example, increasing pressure can enhance flow rates but may lead to fouling, where materials clog the membrane. Monitor the system regularly to identify when cleaning is necessary.
Tips: Always pre-filter your solution to remove larger particles. This action protects the membrane and extends its life. Ensure that the ultrafiltration system is properly calibrated. A well-calibrated system will perform better. Lastly, consider the concentration of your feed solution. High concentrations can lead to concentration polarization, which can impact separation quality. Regular checks can prevent these issues.
Laboratory ultrafiltration is a powerful technique for separating substances. The effectiveness of this process largely depends on the type of membranes used. Several membrane types are available, each serving a specific purpose.
Polymeric membranes are the most common choice. They are flexible and can withstand various chemical environments. These membranes can filter out larger molecules while allowing smaller ones to pass through. They work well for biological samples and are relatively easy to handle. However, they may not be suitable for all solutions, and some can clog over time.
Ceramic membranes offer durability and resistance to high temperatures. They are effective at separating proteins and nanoparticles. Their rigid structure helps prevent fouling, but they can be expensive. The selection of membrane depends on the application goals. It's crucial to consider compatibility with the solution.
Membrane performance can be inconsistent. Factors such as concentration and pressure affect efficiency. Regular maintenance and periodic assessments are essential. In some cases, the chosen membrane might require reevaluation. Ensuring optimal results involves continuous learning and adaptation in membrane technology.
Setting up an ultrafiltration system requires careful planning. Begin with selecting the right membrane based on your specific application. For example, some membranes can retain solutes greater than 5 kDa, while others are suited for smaller molecules. It is crucial to review technical data. According to recent industry reports, a poorly chosen membrane can lead to a 20% decrease in efficiency.
Next, prepare your sample by ensuring it is free of large particles. Use a pre-filter to eliminate any unwanted debris. This step is often overlooked, yet it can significantly improve the lifespan of your membrane. Research indicates that contamination can reduce filter performance by up to 50%. It’s essential to keep your workspace clean and organized, which can help avoid operational errors.
When configuring the system, connect the components securely. Pay close attention to pressure settings. Incorrect pressure can cause membrane fouling. Regular monitoring is necessary. Studies have shown that consistent monitoring can enhance system efficiency by as much as 30%. Be aware of signs of membrane wear, as they may require reflection on your procedures. Identifying issues early can save time and resources in the long run.
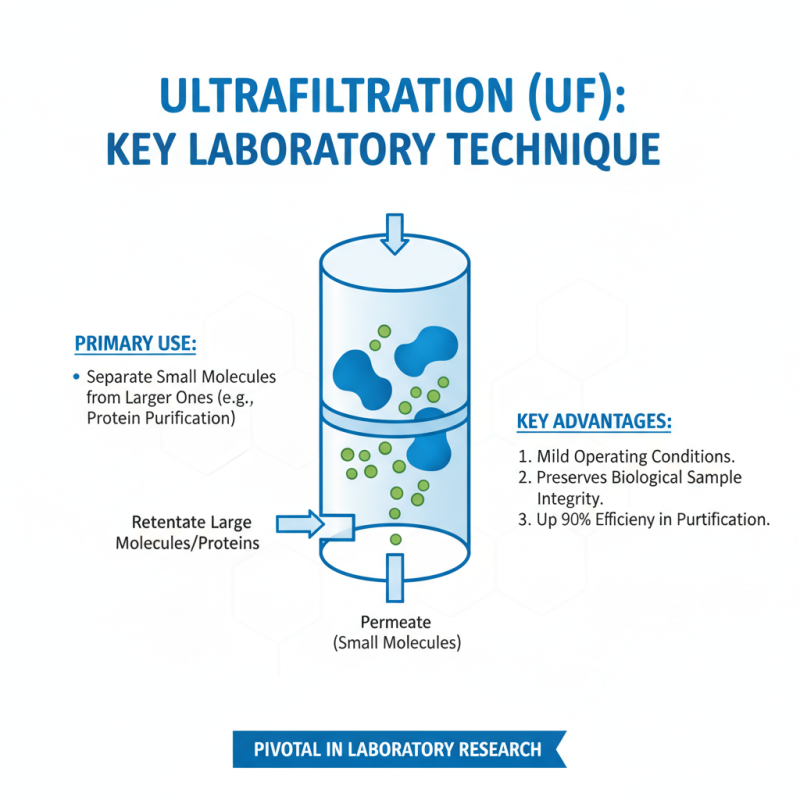
Ultrafiltration (UF) has become a pivotal technique in laboratory research. This method is widely used to separate small molecules from larger ones. It operates effectively under mild conditions, preserving the integrity of biological samples. According to a recent industry report, ultrafiltration can achieve separations with up to 90% efficiency in protein purification.
One key application is in the field of biotechnology. Researchers utilize UF to concentrate proteins and remove contaminants. This process is vital in producing high-quality samples for further analysis. For instance, in vaccine development, ultrafiltration helps isolate antigens while removing undesired components. The throughput can reach significant levels, often exceeding 1 liter per hour for large-scale applications.
Another important area is environmental monitoring. Ultrafiltration is used to detect pollutants in water samples. The technique can effectively filter out suspended particles and microorganisms. However, it may not completely remove dissolved substances. This limitation necessitates further refinement in methods. The need for continuous improvement is evident in achieving better detection limits for trace pollutants. Thus, while ultrafiltration is a powerful tool, it still requires critical evaluation to enhance its effectiveness in various applications.
Ultrafiltration is a powerful technique for separating macromolecules. However, common issues can often hinder efficiency. Clogging is one major problem. This occurs when particles accumulate on the membrane surface. It can lead to reduced flow rates. Regular maintenance helps prevent this buildup. Check for clarity in your feed solution to minimize fouling.
Another issue is poor permeate quality. This might happen due to improper membrane selection or operating conditions. Ensure that the membrane's pore size aligns with your specific separation needs. Monitor the operating pressure periodically. This helps to maintain optimal performance.
Tips: Always maintain your equipment. Regular washing can keep membranes clean. Additionally, consider scaling up your solution for bigger experiments. Understanding your process can save time and resources. Optimization often requires trial and error. Documenting each run can help identify patterns and solutions.