Chengdu UCELLO Biotech’s UC101 Receives FDA IND Approval, Supported by Challenge IM’s Cutting-Edge Ultrafiltration Technology
Chengdu, January 2025– Chengdu UCELLO Biotech Co., Ltd. has recently announced that its self-developed CD19-targeted chimeric antigen receptor (CAR) allogeneic universal T cell therapy, UC101, has received approval from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) application on January 11, 2025. This approval marks UC101 as the first allogeneic universal CAR-T product worldwide to secure an FDA IND.
As the exclusive supplier of ultrafiltration process equipment for the UC101 project, Challenge IM delivers a fully automated cross-flow filtration system to Chengdu UCELLO, ensuring the smooth operation of the project’s critical processes.

About Challenge IM
Dedicated to the development and application of membrane filtration process products in the biopharmaceutical industry, Challenge IM is a high-tech enterprise integrating R&D, manufacturing, sales, and technical services. Its products are widely utilized in various stages of drug development, including antibodies and antibody-drug conjugates, cell and gene therapies, vaccines, and nucleic acid drugs. The company's ultrafiltration product series spans from trial and pilot scales to full production levels.
Recognized as a National High-tech Enterprise, Challenge IM holds over 30 patents. Notably, its fully automated, unmanned tangential flow filtration system, with exclusive patents, fills a critical gap in both domestic and international markets, driving global technological innovation.
Guided by the belief of "Challenge IM: Make Achievements Attain," the company focuses on producing outstanding products, nurturing talented professionals, and helping customers become industry leaders. Challenge IM has established strong partnerships with leading clients in specialized industries, such as GenScript, WuXi Biologics, BeiGene, Innovent, Sinopharm, and many others.





 Tangential Flow Filtration(TFF) System
Tangential Flow Filtration(TFF) System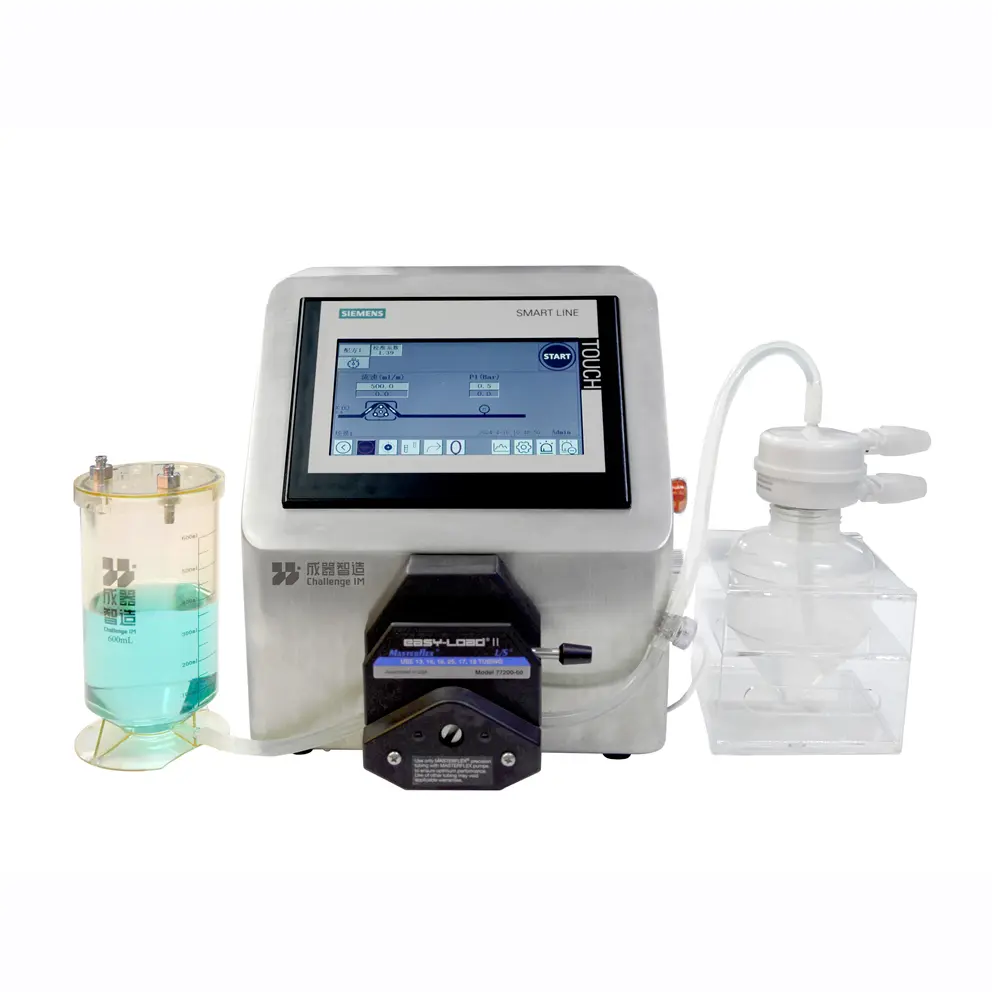 Jump Pump
Jump Pump FIT Integrity Tester
FIT Integrity Tester High Pressure Homogenizers
High Pressure Homogenizers Microfluidics
Microfluidics






